Electricity and clean water -things that we easily have access to are unfortunately luxuries for those in underdeveloped countries.
In fact, not only is there a lack of resources in third-world countries, but also the whole world is facing energy crisis and water pollution. My objective is to find an eco-friendly and economical approach to solve both issues.
My device H2PRO relies on photocatalytic reactions to purify and sterilize wastewater and to generate electricity using hydrogen produced. This sustainable process only requires titania and light. What’s more is that organic pollutant doesn’t only get decomposed but will also enhance the reaction rate.
It is composed of 2 parts –the upper unit for photocatalytic water-purification and hydrogen-generation, which is connected to a fuel cell and the bottom unit for further water filtration.
H2PRO’s feasibility in the removal of organic pollutant was examined to be excellent-almost 90% of organic compound was decomposed after 2hours. However, its performance in electricity generation was quite unstable although theoretically and experimentally the photocatalytic hydrogen yield is proved to be satisfactory and even better in the presence of organic pollutant. I will keep improving this device until stable electricity generation is achieved.
In conclusion, I have successfully introduced a design for a portable electricity-generation and water-purification unit. Generally speaking, H2PRO has demonstrated its potential to feasibly provide clean water and sustainable energy to the needy ones. I will keep "practicalizing" the electricity-generation unit so that people can really benefit from my design one day.


ABOUT ME
Hello! My name is Cynthia Lam. I go to Balwyn High School in Melbourne, Australia. Besides Science, I love travelling, music and making crafts.
I am always fascinated by the how scientists put imaginations into practice to change the world. Rita Levi-Montalcini's perseverance in science is truly an inspiration to me. Yet, although there was always a spark of passion for science in my heart, I always thought that I was too young for researches and inventions - until last year.
In April 2013, out of curiosity and my love for Chemistry, I started my first research on Titania's Photocatalysis. I investigated the optimum conditions for photocatalytic hydrogen generation and it won the Major Bursary in Victoria's Science Talent Search. It was a very rewarding and exciting experience to complete a research, I was hence motivated to challenge myself and to create a helpful device that puts what I investigated into practice.
I would like to study Medicine or Environmental Science in the future because I want to be able to help those in need. By creating H2PRO, I introduced an eco-friendly alternative for providing people in underdeveloped countries clean water and electricity. There is still a long way to go, but I am glad I took my first step to make a difference. Winning would mean a motivation for me to keep improving my device and to bravely follow my dreams. Hopefully it would also raise the awareness of the importance of clean water and electricity in underdeveloped countries.
QUESTION / PROPOSAL
Is it possible to create a portable device that purifies wastewater while generating electricity sustainably and affordably?
This question is raised not only because of the lack of clean water and electricity in third-world countries, but also because we are all facing energy crisis and water pollution. My objective is to find an eco-friendly and economical approach to solve both issues.
Based on what I developed from investigating photocatalysis, I aim to create a device that can put the mechanisms into practice. In photocatalysis, not only water is purified and sterilized, but hydrogen is also produced through water-splitting, which can be used to generate electricity.
The entire sustainable process only needs titania and light-no additional power source is required. However, hydrogen production is generally low since photoexcited electrons tend to fall back to the hole(i.e.photoinduced electron-hole combination). Fortunately, it can be overcome by adding reductants, while some organic pollutants serve such purpose. Hence, I propose to combine the two mechanisms together to enhance the yield and lower the cost of hydrogen generation, meanwhile efficient water purification can also be achieved.
There are similar designs existing, but they require either an excessively complex use of technology or an external energy source, which means they are not sustainable, affordable and manageable for users in underdeveloped countries. Nonetheless, I hypothesize that photocatalysis can be applied in a manageable scale that allows water purification and electricity production to be economically and sustainably performed in a portable device as well as at a household level.
RESEARCH
EXISTING WORK
There is an existing design for mobile hydrogen energy and water supply, although feasible, that design needs an external solar power source to purify water and produce hydrogen through electrolyzer, which makes it costlier and less efficient. This inspires me to create a self-sustainable portable device that can purify wastewater and produce electricity through only photocatalysis (without any additional electricity source).
METHODOLOGY
I. Photocatalytic Redox Reactions
When titania absorbs ultraviolet energy, which is comparable to its band gap, photoexcitation of electron takes place and an electron-hole pair is produced.
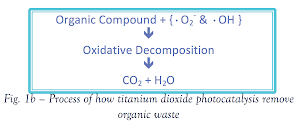
Reduced by the excited electron, oxygen forms super oxide anion (•O2-). The hole reacts with water producing hydroxyl radicals (•OH).
The radicals yielded in the process oxidize organic compound, resulting in water purification. Furthermore, organic compounds may also be oxidized by the hole. In either case, the organic compound decomposed to yield harmless CO2 and H2O.
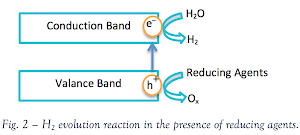
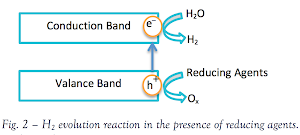
I. Photocatalytic Water Splitting
The
effect of water splitting using titania was first discovered by Akira
Fujishima. The photoinduced electrons and holes cause redox reactions
similarly to electrolysis, causing water molecules to be reduced by the
electrons to form hydrogen and oxidized by the holes to form oxygen
(most oxygen is consumed in side reactions so hydrogen is the major
product).
III. Recombination of the photoinduced electron-hole pairs
Electrons tend to move to lower energies. When this transfer occurs from band to band, it is called recombination.
In
this process, an electron (e–), which has been excited from the valence
band to the conduction band of titania, falls back into an empty state
in the valence band, which is the hole (h+).
This
phenomenon hinders photocatalytic redox and water-splitting reactions.
Hence, preventing the recombination reaction had long been the
centerpiece of the field.
Different
attempts to hinder the recombination of electron-hole pairs have been
proposed; doping titania with noble metals for example. The addition of a
sacrificial agent – a reducing agent or an oxidizing agent – is known
as an excellent approach to overcome such limitation. When
photocatalysis takes place in an aqueous solution including a reductant,
a.k.a. electron donors/ hole scavengers, photoinduced holes
irreversibly oxidize the reductant instead of water. Therefore, hydrogen
production is enhanced.
Lots
of common organic pollutants such as methanol, glycerol and EDTA can
act as excellent reductants. As hole scavengers, they minimize the
chance of recombination of the photoinduced electron-hole pairs, hence
enhancing the rate of hydrogen generation. At the same time, they can be
effectively decomposed.

III. Hydrogen Fuel Cell
There
are various fuel cells, but generally they work similarly. Hydrogen
fuel cell has an anode and a cathode separated by a membrane. Oxygen
passes over cathode and hydrogen over anode.
H2 reacts to a catalyst on the anode that converts H2 into electrons and hydrogen ion.
The
mobile electrons travel through a wire creating the electric current.
The hydrogen ions move through the electrolyte membrane to the cathode
where they react with oxygen and electrons to form water.
ORIGINAL: Google Science Fair

No hay comentarios:
Publicar un comentario
Nota: solo los miembros de este blog pueden publicar comentarios.